Keys to successful commercialization
A new drug product launch is an exciting time for a pharmaceutical company. New product development is typically a critical organizational priority, with high complexity and diverse stakeholders. Therefore, when the long, difficult, and costly development process is finally coming to fruition, hopes are high, and the pressure is on for successful commercialization.
However, the launch process is fraught with its own set of struggles: increased regulation, market access barriers, low-coverage rates, supply chain challenges, and many other risks. If mishandled, these can result in missed opportunities for patients and loss of market share to competitors.
Integrating project, program, and portfolio management leadership into the product commercialization life cycle can help ease these challenges by ensuring all required efforts are identified, prioritized, assigned, planned, and managed as part of a comprehensive drug launch program. The following are key areas that are vital to successful commercialization:
Management of cross-functional interactions
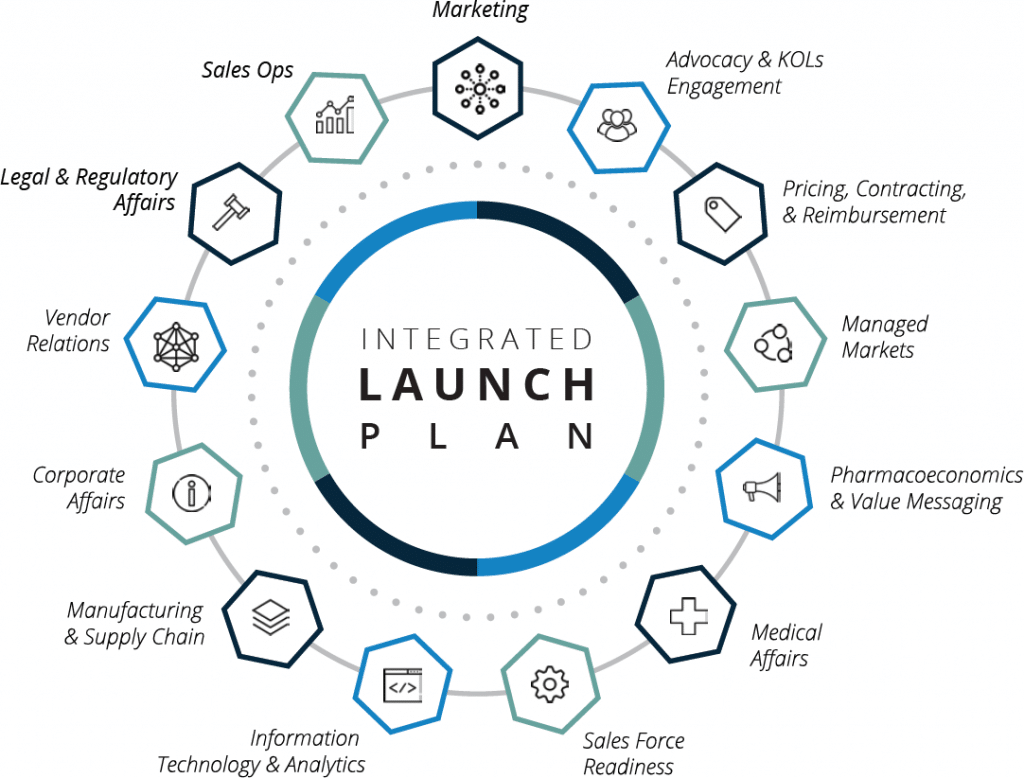
Developing and managing a detailed, comprehensive commercialization plan to orchestrate the cross-functional activities (sometimes for multiple organizations) required to execute the launch; and eliminating silos among regulatory, legal, advocacy, medical affairs, R&D, sales, marketing, manufacturing, supply chain network, customer support, and other involved business functions.
External stakeholder management
Managing the efforts of a large and diverse collection of stakeholders and the flow of information among them; and focusing on the triad of the patient, healthcare provider (HCP), and payer landscape.
Strategic alliance & partnership management
Coordinating efforts, managing complexities, and avoiding potential misalignments with launch partners, including those with shared product ownership.
Scope & schedule management
Developing processes to ensure that the launch project’s scope and corresponding schedule are accurately defined and mapped; managing activities pursuant to schedules to keep the launch on track for regulatory approval and to promote predictability of activities; and promoting understanding among stakeholders about individual responsibilities for detailed project deliverables and defining success factors.
Risk management
Minimizing risks to avoid issues and proactively defining contingency plans before risks occur.
The following is a closer examination of how each of these five elements plays out in real-world situations.
 MANAGEMENT OF CROSS-FUNCTIONAL INTERACTIONS
MANAGEMENT OF CROSS-FUNCTIONAL INTERACTIONS
Product commercialization processes involve complex multi-departmental (and often multi-organizational) collaboration to establish market access, patient and provider awareness, sales force readiness, customer support programs, supply chain preparedness, and more. Coordination becomes even more complex when working with fully remote teams, or when the drug is being developed in partnership between two companies.
Using project management to plan and lead the activities of cross-functional teams is a core competency for all phases of the drug development life cycle, including execution of a product launch. Without comprehensive planning and dedicated project leadership, team members and their contributions can become siloed, creating delays or even failures to commercialize a new drug.
A robust patient-centric life cycle strategy must include an integrated global regulatory framework from the beginning. For optimal efficiency, regulatory and commercial operations must work closely together to meet the patient and business needs. Clinical, economic, and marketing deliverables at each stage of product development from pre-approval through commercialization must adhere to legal and regulatory requirements, processes, and internal company policies to optimize quality and regulatory best practices.
Understanding the roles and responsibilities among the different functions of the drug launch is critical to ensuring alignment and cooperation. This requires comprehensive planning, effective communication, and change management leadership to ensure the performance of a winning commercial team through the launch of the new drug.
Often, pharmaceutical companies lack some of the required expertise or resources necessary to execute a drug launch. Proactive identification of resource requirements and resource gaps is part of the launch planning process. External resources and partnerships with vendors of specialized expertise or contract manufacturing organizations (CMOs) must be leveraged as necessary to fill talent gaps on the pharmaceutical company’s internal commercialization team.
 EXTERNAL STAKEHOLDER MANAGEMENT
EXTERNAL STAKEHOLDER MANAGEMENT
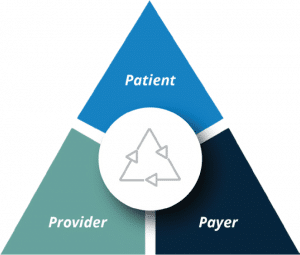
Pharmaceutical companies must not only overcome the challenges of discovering and developing innovative new therapies; they must also define and execute strategies that will provide patients with access to the drugs they require. These market access strategies must include communication on the benefits of the drug to external stakeholders, establish pricing and reimbursement programs, and conduct prescription fulfilment. A robust market access program must be created and orchestrated with a focus on the stakeholder triad of patient, healthcare provider, and payer landscape of health insurers, pharmacy benefits managers (PBMs), and formulary decision-makers. With the increasing demand to promote real world evidence (RWE) and outcomes vs. therapies, a comprehensive pull-through communication system is crucial to enable ongoing measurement of the related key performance indicators and analytics.
As a patient-centric culture becomes more prevalent in the pharmaceutical industry, improved processes will play a bigger role in managing the Co-creation consumer programs will provide unique insights for successful execution that influences HCPs and payers along the adoption ladder for a new therapy. For example, as part of the launch readiness of a new drug, a specific patient population must be defined and characterized during the development of commercial strategic initiatives and tactics. Alignment on the drug’s clinical value and messaging should be well coordinated across the pharmaceutical organization and with external stakeholders.
Just as the healthcare provider plays a critical role in shaping the patient journey, integrated program leadership ensures the execution of the critical market access initiatives to optimize the patient journey. Such initiatives can include:
- Understanding patient behaviors allows the pharmaceutical company to support the healthcare provider with appropriate market access methods, including the communication of the drug’s clinical value based on results-driven outcomes such as personal/nonpersonal promotion, key opinion leader (KOL) engagement, advocacy, etc.
- Early development of an effective integrated supply chain management infrastructure is critical for planning market access activities and implementation throughout the multichannel entities, including integrated delivery networks (IDNs), accountable care organizations (ACOs), and specialty pharmacy providers.
- The creation and management of customer service and support programs, such as nurse programs, copay, and field reimbursement support, are also essential to a comprehensive market access program and product launch.
Payers and formulary decision-makers are increasingly important when introducing a new drug or drug class. Pricing, reimbursement authorities, and formulary decision-makers demand evidence of a drug’s cost effectiveness and use this to make pricing and purchasing decisions. The complexities surrounding dedicated reimbursement, pricing, and contracting platforms are critical drivers in the launch planning, especially for innovative drugs such as biosimilars and injectables. Project management and resource planning can help manage the complex payer landscape by creating steps toward the goal of secure market access and commercial success.
Early inclusion of pharmacoeconomic measurements in the drug development process (i.e., Phases II–III) can help identify drug candidates that might not demonstrate cost effectiveness, achieve market access, or meet reimbursement goals.
Early availability of such information can also be a factor in determining pricing options and making go/no–go decisions for Phase III and Phase IV investments. Developing pharmacoeconomic and scientific medical liaison communication strategies is a critical component of the launch plan and can help pharmaceutical companies articulate meaningful discussions with payers and shape the external environment for better utilization management (i.e., budget impact tools, Food & Drug Administration Modernization Act Section 114 materials, and Pre-approval Information Exchange).
Finally, the use of third-party contract manufacturing organizations (CMOs) adds complexity, as the successful commercialization of the product may not be the focus of both organizations. The product owner is looking for product that meets specifications, on time, and in the desired quantity. The CMO must balance this with any contractual obligations to other organizations. For this reason, the success of the commercial launch depends on the successful identification, contracting and managing of a CMO to limit risk.
 STRATEGIC ALLIANCE & PARTNERSHIP MANAGEMENT
STRATEGIC ALLIANCE & PARTNERSHIP MANAGEMENT
Current industry trends show an increase in the number of co-developed and co-commercialized products. These joint ventures or partnerships allow for the optimization of resources for shared objectives. In some cases, the co-development strategy allows an organization with a deficiency in expertise in one functional area be supported by a partner with the skills needed.
Whether it’s an alliance or partnership is with an academic institution, government agency, healthcare system, or other pharmaceutical company—or a consortium of multiple of these—it requires an added level of coordination. Not only are there additional stakeholders to manage, they may have different motivations or priorities. Gaining alignment on scope, as detailed in the following section, will be critical to achieving a successful launch.
Many partnerships are formed to multiply forces in research and development, and others are formed later in the life cycle. Regardless. the management of cross-functional work is further complicated by multiple active sales forces, marketing departments, regulatory teams, etc.
 SCOPE & SCHEDULE MANAGEMENT
SCOPE & SCHEDULE MANAGEMENT
Each product launch has a unique set of goals, objectives, and life cycle characteristics, all of which must align with the organization’s (or organizations’) overarching goals and strategic imperatives. Balancing the specific commercial operation efforts with the overall strategy and vision is critical to the product launch scope definition, success criteria, and baseline. It also allows the market access team to focus on developing the most critical tools and resources. An iterative monitoring and controlling process of the project scope baseline will yield better outcomes and strategic alignment.
The scope and interdependencies involved in a new drug launch must be controlled and monitored throughout the project life cycle to ensure focus and avoid scope creep and schedule delays. Comprehensive delineation of the launch scope of work, a corresponding integrated launch schedule, and a change control process are critical for success. These items make up the integrated drug launch plan, which is managed by the project leader in collaboration with the full cross-functional commercial launch team, applying acceptance criteria and work breakdown structure (WBS) methodologies. This plan enables project leaders to monitor success and regularly communicate status, interdependencies, and progress.
Competing priorities and ideas within the organization should be constantly monitored with respect to the launch’s established goals and return on investment. For instance, the marketing team develops tools to support the sales force and field representatives in driving demand and targeted sales/brand performance. The project/program leader guides the organization and identifies and prioritizes the must-have tools and resources according to the commercialization plan. Additional platforms and tools that were not part of the initial plan should be vetted through the integrated change control process.
As the marketplace and technology continually change–and change more quickly due to the pandemic and its fallout—decisions and strategies must be amended and reprioritized. It is important for the team’s productivity and morale to work on the right objectives. With many moving parts, effective prioritization and communication help people understand the strategy to ensure that individual trade-offs are made properly. Effective prioritization and communication also help a team respond more rapidly to changes in the company’s environment.
 RISK MANAGEMENT
RISK MANAGEMENT
While most drug development and commercial operations teams are excellent at problem solving, a successful project leader helps move the team from “problem solving” to “problem searching” as a method of continuous risk management. Regularly identifying, documenting, and reviewing critical risk sources—including regulatory, operations, team members’ capabilities and availability, functional siloes and cultural challenges, and external market events—helps the launch team focus on the required risk avoidance alternatives, contingencies, and appropriate responses. A comprehensive risk management plan is instrumental in contributing to predictable progress toward defined goals.
The risk assessment can become even more complex in the case of a new drug class. Weighing the appropriate investment for pre-launch and post-launch preparedness initiatives is crucial. Launch program risk management is a continuous process that includes identification, analysis, response planning, and mitigation. Implementing a robust risk management plan helps the pharmaceutical organization quickly react to “predicted” risk scenarios and capitalize on potential opportunities, such as:
- Changes and developments in the regulatory approval process;
- Increased competition from branded and generic drugs; and
- The level of acceptance from patients, KOLs, and payers to the new drug.
By scenario planning and anticipating different outcomes throughout the life cycle of the product launch, the project leader helps the cross-functional team increase the level of success.
 Conclusion
Conclusion
A successful product launch and post launch commercialization depend on the strategic implementation of fully integrated project, program, and portfolio management into the product commercialization life cycle. However, while most pharmaceutical companies have talented functional resources, organizations often lack the dedicated expertise and discipline needed to lead the matrix structure and interdependencies essential to the success of a commercialization program.
The value of project, program, and portfolio leadership for drug launch and commercial operations has become increasingly evident as more pharmaceutical organizations leverage this expertise to maximize organizational capabilities and capacity to launch new drugs with speed, efficiency, and effectiveness. Implementing such expertise enables the pharmaceutical company to transform its vision into an actionable roadmap, compress time to market, and streamline operations to accelerate benefits for the patient and value for the company.
Service: Product Development
Industry: Life Sciences
Authors:
Eyal Golan
Senior Project Management Consultant
Ian Pudge
Senior Project Management Consultant
Integrated Project Management Co.
The Pandemic’s Impact on Commercialization
COVID-19 has had a global impact on all aspects of life. Virtual work environments, deferred clinical trials, changing regulatory approval pathways, and supply chain and production limitations have led to increased complexity for the commercialization of new drug products as well.
Manufacturing, logistics, and customs bottlenecks have eased since the onset of the pandemic. And while it continues to expedite COVID therapies, vaccines, and tests, the FDA has maintained its approval pipeline for other drugs.
What has not returned to “normal”—and perhaps never will—is face-to-face access to colleagues, external partners, HCPs, and patients. It is projected that travel and large conferences may be reduced for years, while large virtual gatherings and advisory boards will continue to rise. Telemedicine and other remote patient access tap a demand for convenience as well as safety, and surely will continue to evolve rather than abate. Many employers have recognized that working from home hasn’t negatively impacted productivity or performance and may even save costs and help retain employees.
Pharmaceutical companies must shift communication methods and use virtual collaboration, digital marketing, and technology to adapt.
From a program management perspective, commercial operation teams must tailor execution appropriately. Leading virtual teams requires a re-emphasis on project management essentials like consistent and effective communication, knowledge management, facilitation, setting clear expectations, and team building.
The continuous uncertainties and narrowed window for a competitive advantage require adopting a flexible, iterative, and agile mechanism. This will allow for efficient decision making and rapid alignment to shifting priorities and continuous improvement of tactics and strategic execution.
Just as managers are using collaboration and conferencing tools to lead teams, sales representatives, marketers, and customer service personnel are using technology to engage and educate physicians, pharmacists, and even patients. For instance, many commercial operations teams are finding it easy and effective to schedule off-hours 30-minute virtual roundtable discussions or information sessions with small groups of HCPs, in lieu of traveling the region to host several nights of dinner discussions.
While meeting virtually with HCPs, marketers are also putting more resources into reaching patients directly. Television and print advertising budgets are up, and websites, patient portals, and other digital tools are being enhanced.
As the situation evolves, indeed in any time of uncertainty, ensuring a product’s commercialization strategy and team operate effectively and efficiently is critical. Integrated project plans with cross-functional alignment still must perform at high levels. Leveraging technology, tools, and skillsets to constantly enhance progress, adapt to new external challenges, and mitigate risks will be paramount.
Start a Conversation
If you are facing challenges across the product development process, IPM’s project management consultants can turn the most complex situations into manageable strategies and plans. By employing an engaged, hands-on approach, we execute your initiatives and lead your teams so that you reach your goals on time and on budget.
Click here to learn how we can put IPM’s years of experience working with some of the largest drug manufacturers to use in your organization. If you have any questions or wish to discuss your project needs, submit the form below to contact one of our expert consultants.
By submitting this form, you agree to receive communications regarding your message. You also agree to receive our quarterly newsletter and occasional messages featuring IPM news and insights. You can opt out anytime. View our full Privacy Policy.